Our Young Scientists Research Experience is one of our favorite programs of the year! Middle and high school students spend a week with us doing their own independent research projects. They come up with a research question, collect data to answer their questions, and share their findings during a presentation to friends and family.
We’re incredibly proud of the work these students did, and we’re excited to share their results with you!
If you’d like to sign up for next year’s Young Scientist Research Experience, you can subscribe to our email newsletter to be notified when registration opens.
Here’s a quick summary of all their hard work:
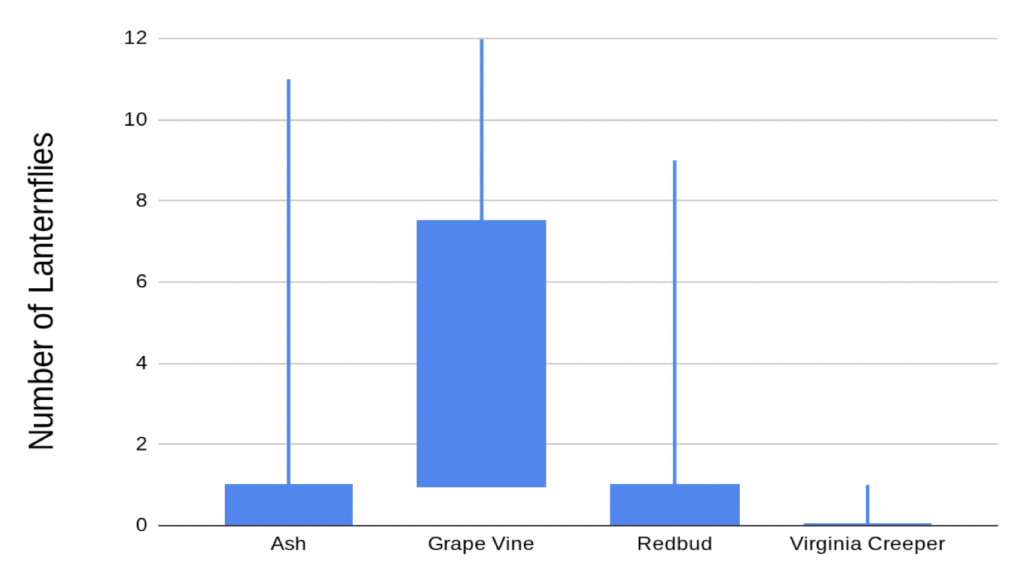
After noticing the many Spotted Lanternflies at Clifton this summer, Alistair, a second time Young Scientist, wondered if there are certain types of plants they prefer over others. Since Spotted Lanternflies are known to use Tree of Heaven and Tree of Heaven has compound leaves, Alistair decided to compare plants with compound and simple leaves to see if the insects might be seeking out leaves similar to their host plants and to compare vines and trees to see if the insects could tell what kind of plant they were landing on. While he didn’t find a big difference between plants with compound and simple leaves, he did find that there were lots of lanternflies on grape vines. Alistair also tried to lure in lanternflies with fake leaves of different colors and textures, but only two lanternflies were fooled. Although the experiment didn’t work, it’s always worth trying a little crafting in the name of science!

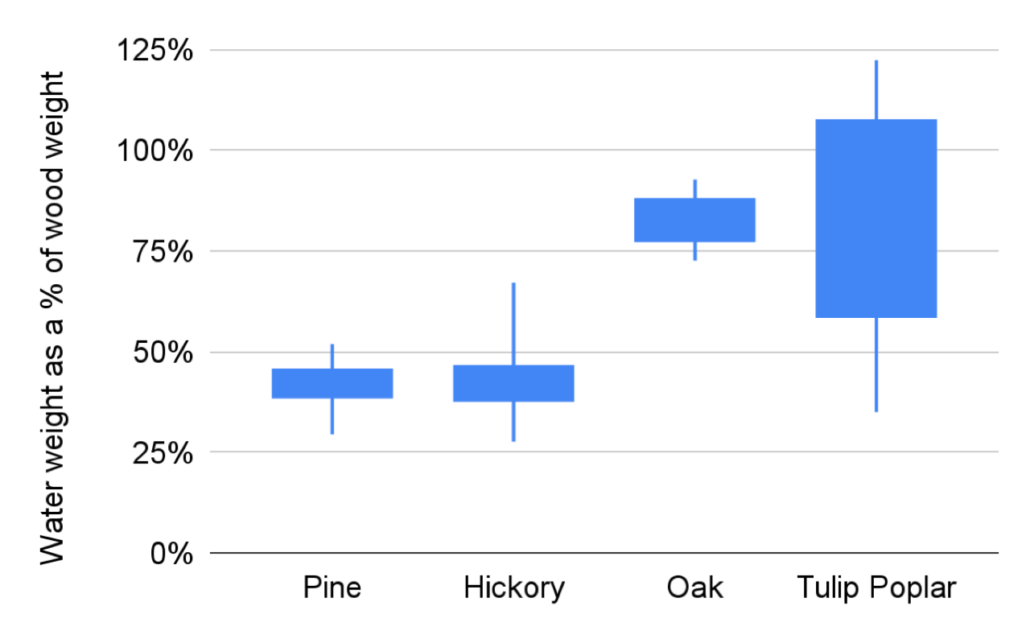
Lane, a fourth time Young Scientist and plant enthusiast, got excited about tree sap after noticing how wet a wood core was on our exploratory hike. He decided to see if different trees had different amount of saps (by measuring how wet their cores were), if different tree saps had different pHs, and if either factor was affected by the soil. Lane found that Tulip Poplars had the most sap by weight and pine trees had the least. Surprisingly, neither the moisture of the wood nor its pH was affected by where the trees were growing. Looking at Lane’s data, what we want to know next is if trees that grow quickly, like Tulip Poplars, tend to have more sap to facilitate that rapid growth!

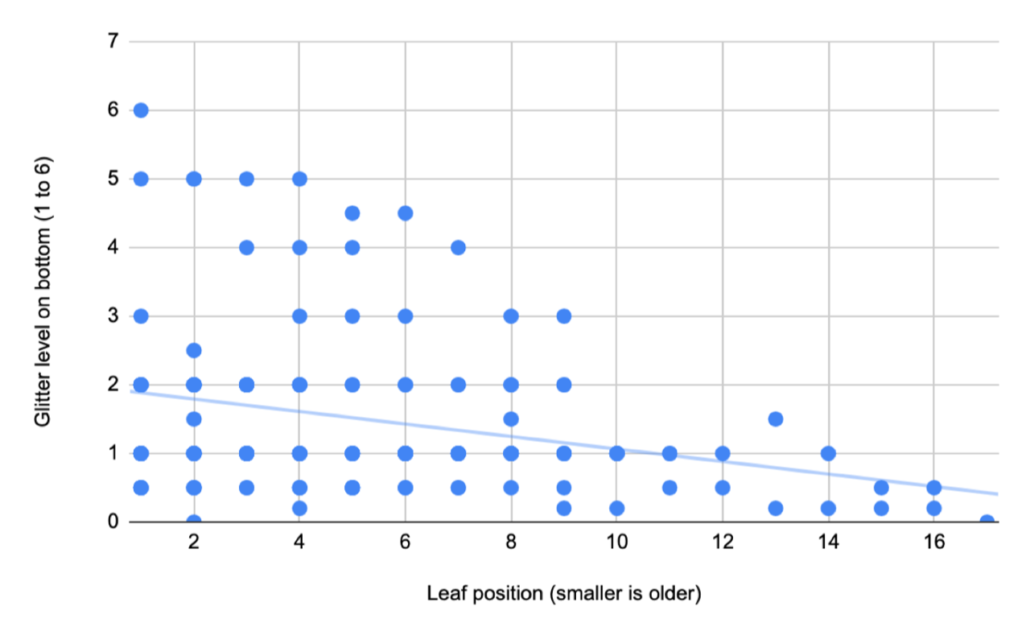
Loreli, a fourth time Young Scientist, chose a question based on her time at our school-year Nature Club. During one Nature Club session focused on nature journaling, Loreli made some close observations of the silvery scales on Autumn Olive leaves and wondered what the point of all that “glitter” was and why some leaves were “glitterier” than others. Based on background research, she came up with four hypotheses: 1. The glitter might help protect the leaves from sun damage. 2. The glitter might help protect the leaves from insect damage. 3. The glitter might help prevent the leaves from drying out. 4. The leaves might lose glitter as they age because it gets shaken off by wind and rain. After measuring the amount of glitter on many leaves and branches, Loreli found no relationship between sun, shade, insect damage, or glitteriness, debunking hypotheses 1. and 2. There was more glitter on the bottoms of leaves, where stomata are, so it may play a role in moisture retention, but surprisingly younger leaves actually had more glitter than older ones! As always, more research is needed!

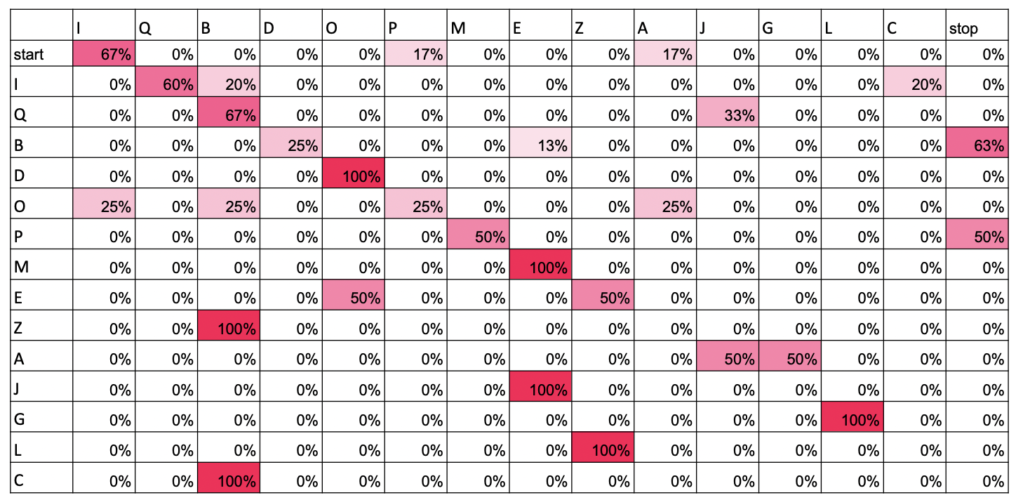
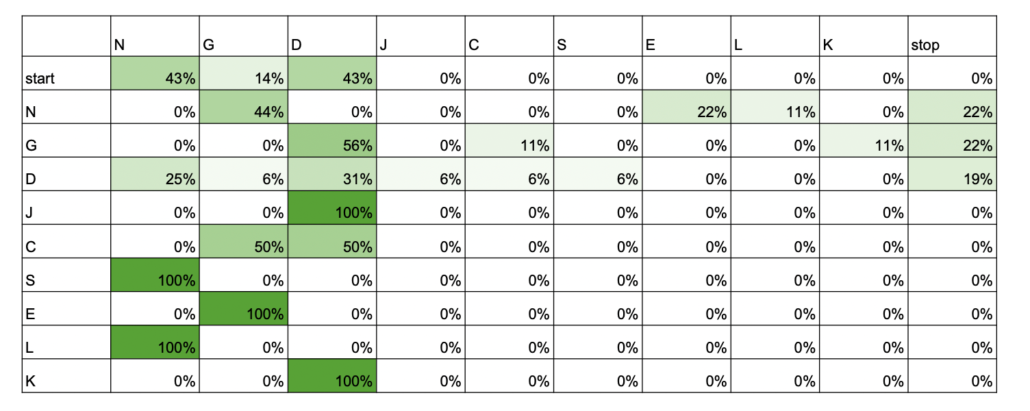
On the hike on the first morning, Luke, an avid birder, got interested in the songs of Red-eyed Vireos. Do they just repeat themselves over and over or do they mix up their songs every now and then? Do different individuals have different songs? When we actually set out to record vireos, we didn’t find as many as we were expecting, so we added Scarlet Tanager to the project, a serendipitous event. Luke made a “library” of phrases for each of the two species and used transition matrices to visualize how individual birds moved between phrases of their songs. Scarlet Tanagers tended to have very stereotypical orders in which they sang their phrases, while Red-eyed Vireos tended to be more random. Does this mean that the two species learn their songs differently? Or that females of the species have preferences for more or less variability? Other highlights of the week were adding Yellow-breasted Chat and Kentucky Warbler to Luke’s “life list.”

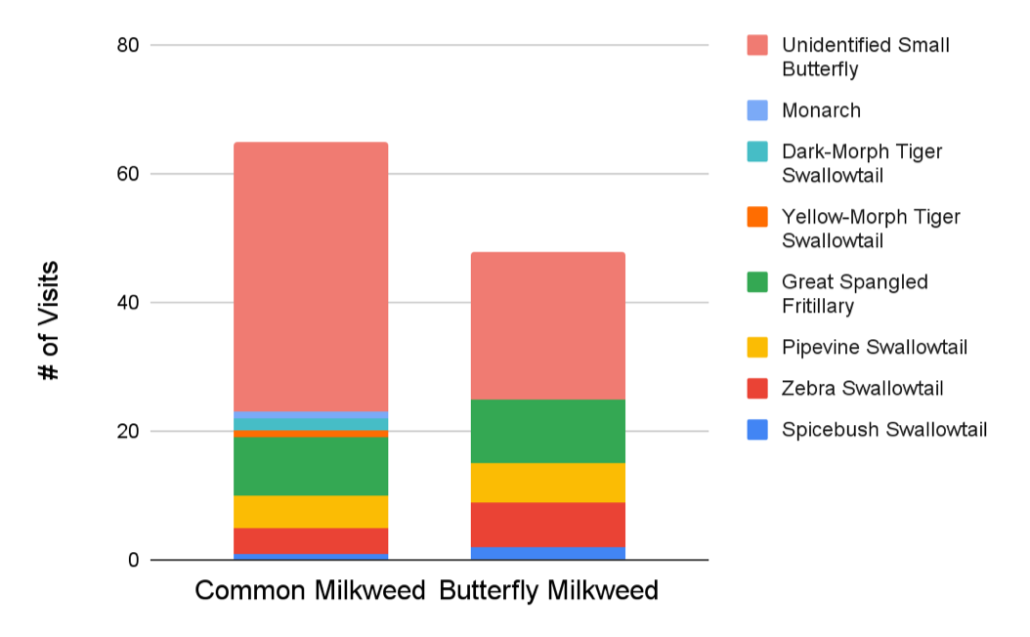
Myla got interested in all the butterflies flying around during a very hot week and wondered what attracted them to different types of flowers. One hypothesis was that they would prefer to hang out on dense patches with lots of flowers. Another is that they would prefer different colors. To test these hypotheses, she compared the butterflies visiting Common Milkweed (pink flowers in dense patches) and Butterfly-weed (orange flowers in sparser patches). Even after controlling for the number of flowers in a patch, butterflies in general seemed to have a preference for Common Milkweed, but there were more big butterflies on Butterfly-weed. Both species of milkweed are very popular, so pick your favorite color and plant it where you can!

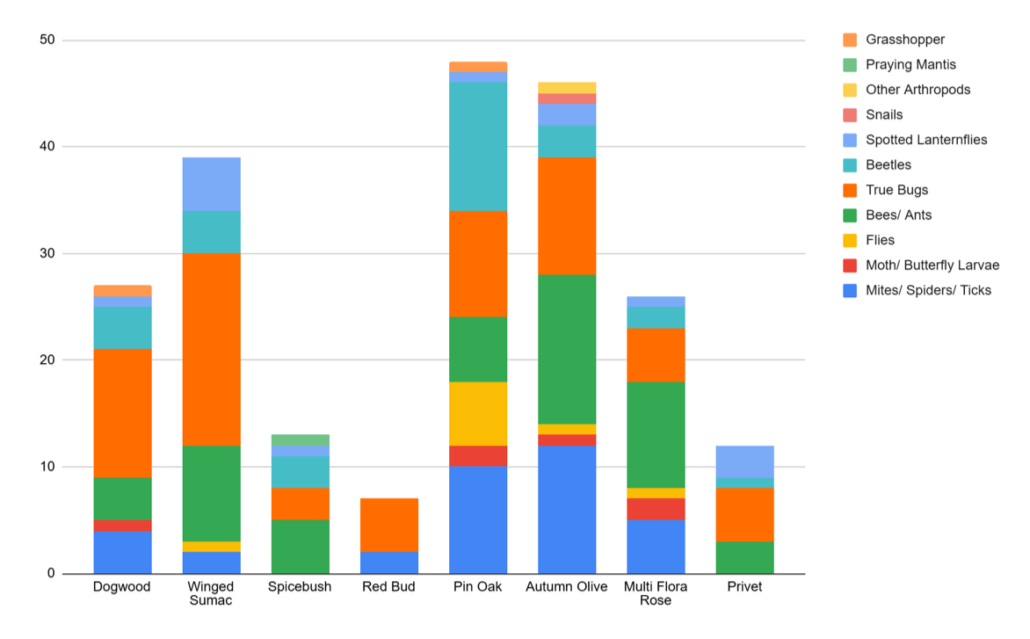
Nevin, a second time Young Scientist and Master Naturalist, is interested in conserving native plants and insects and wanted to study whether insects do indeed prefer native plants, as one would expect. He spent the week beat-sheeting different species of shrubs and counting the numbers of different orders of arthropods that fell off. Pin Oaks, a native species, did indeed have the most bugs, but surprisingly so did a few non-native species of plants. Nevin suspects that specialists insects, like caterpillars, may not have been captured very well with his study methods and that doing the same study at a different time of year would yield different results. One lesson learned was that there were a lot of ticks falling off of Redbuds so beware where you stand!